Most frequent complaints reported(multiple complaints per patient are possible)
Adverse effect may be due to disease and/or other underlying condition and medication/treatment may not be causative factor
DRUG RATING DETAILS (RANK BASED ON RATINGS OF SIMILAR DRUGS; SEE TABLE AT BOTTOM)
| Drug Brand Name | Herceptin |
|---|---|
| Drug Substance/Generic Name | trastuzumab |
| Number of Patients Complaining | 17,906 |
| Serious Reactions to Substance | 18,083 |
| Non Serious Reactions to Substance | 1,409 |
| Substance Not Effective Complaints | 322 |
| At-Ray Ratio (NSR/SR); Safety indicator, higher is safer | 0.08 |
| PP Ratio (Patients Complaining/Not Effective), higher is more effective | 55.61 |
| Drug Rating = PP Ratio* At-Ray Ratio | 0.71 |
Summary (from Wikipedia)
Trastuzumab, sold under the brand name Herceptin among others, is a monoclonal antibody used to treat breast cancer. Specifically it is used for breast cancer that is HER2 receptor positive. It may be used by itself or together with other chemotherapy medication. Trastuzumab is given by slow injection into a vein and injection just under the skin. Common side effects include fever, infection, cough, headache, trouble sleeping, and rash. Other severe side effects include heart failure, allergic reactions, and lung disease. Use during pregnancy may harm the baby. Trastuzumab works by binding to the HER2 receptor and slowing down cell duplication. Trastuzumab was approved for medical use in the United States in 1998. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The wholesale price in the developing world is between 1,800 and 1,955 USD per 440 mg vial. In the United Kingdom a 150 mg vial costs the NHS 407.00 pounds.
Indications and Usage (from FDA label)
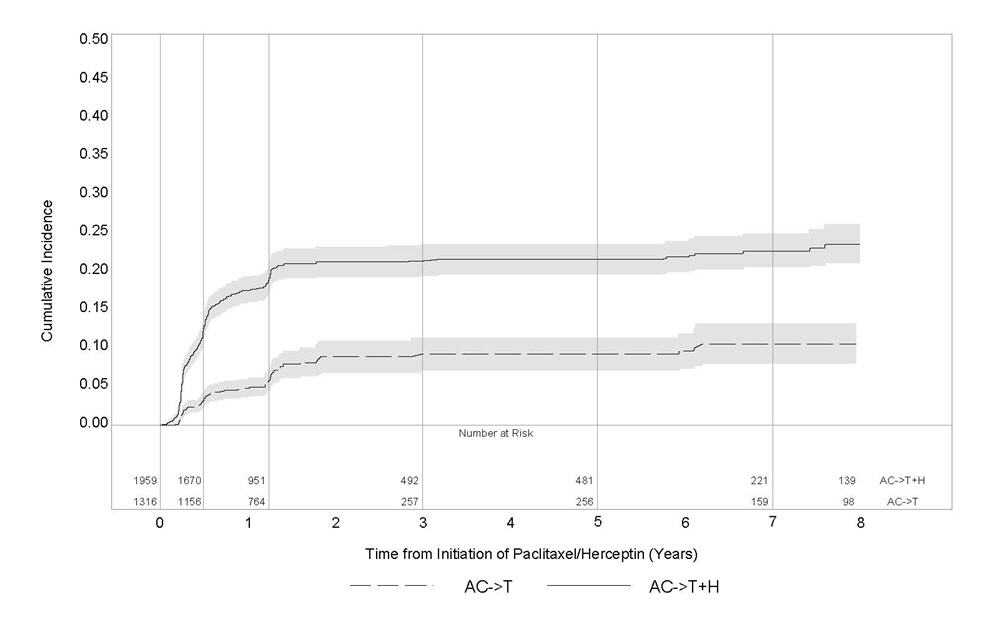
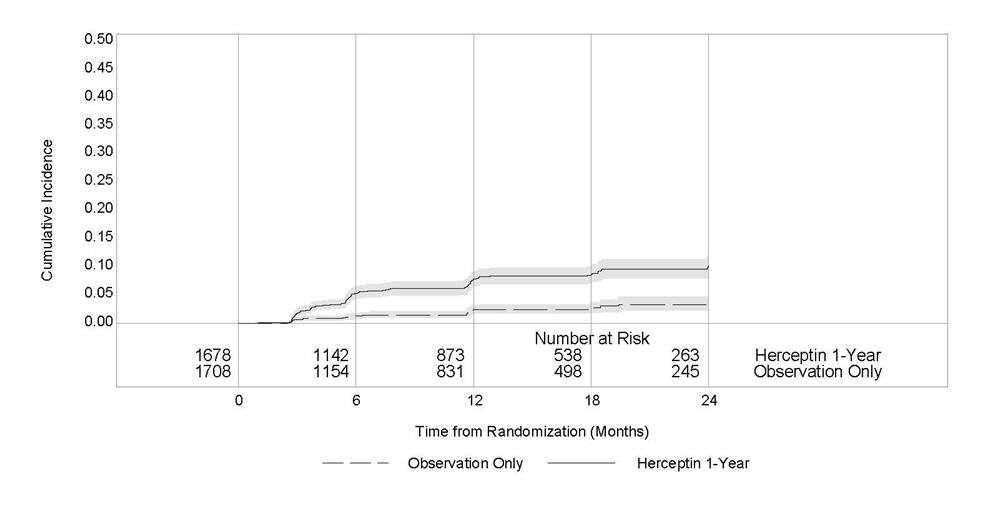
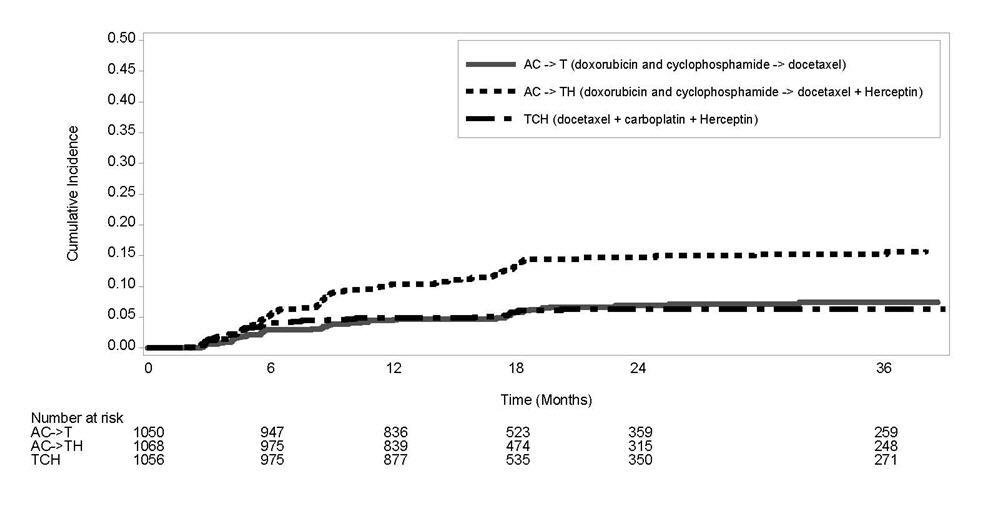
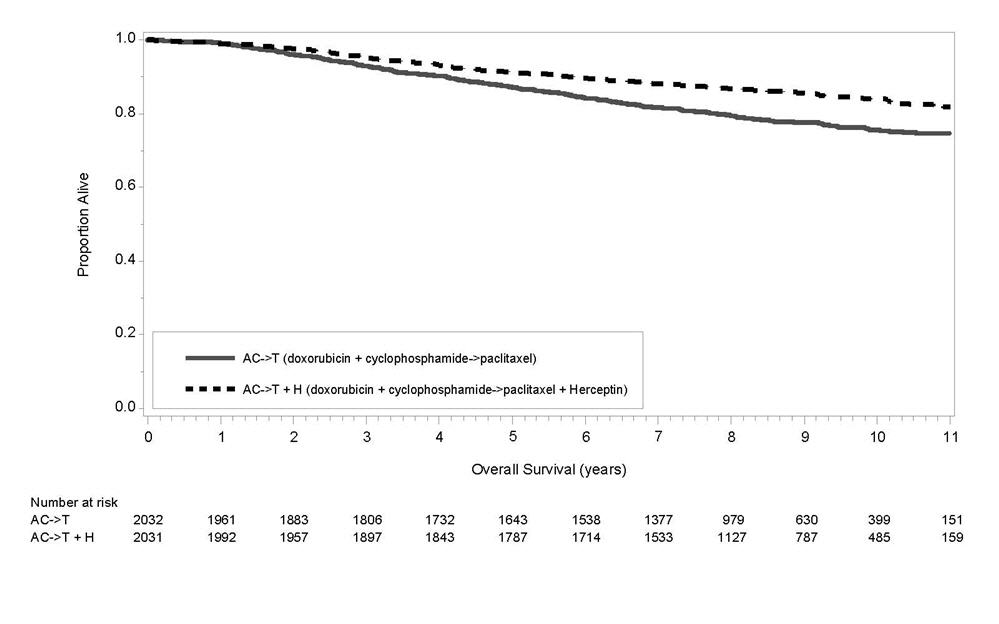
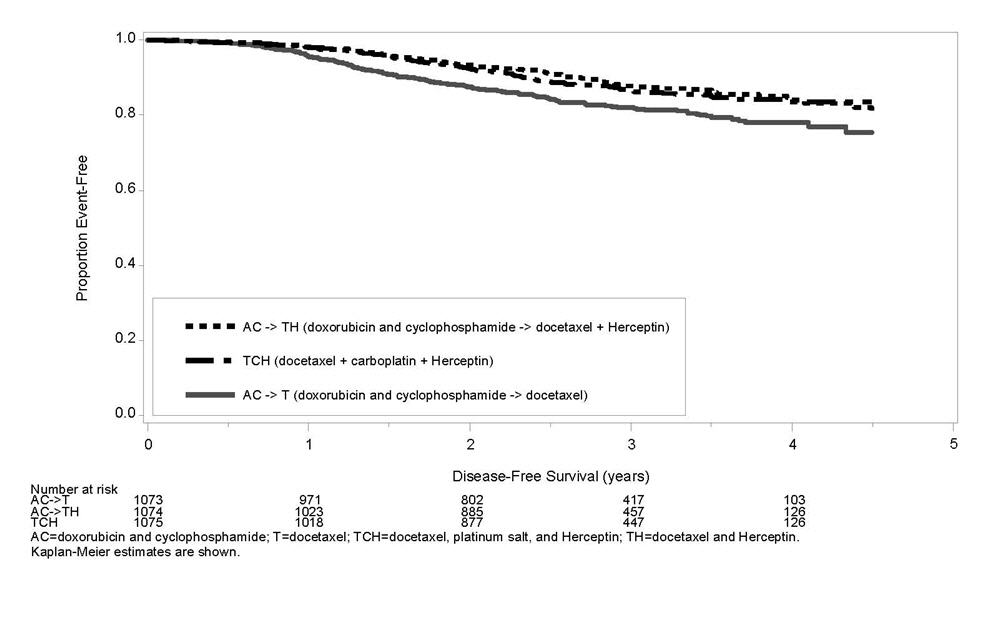
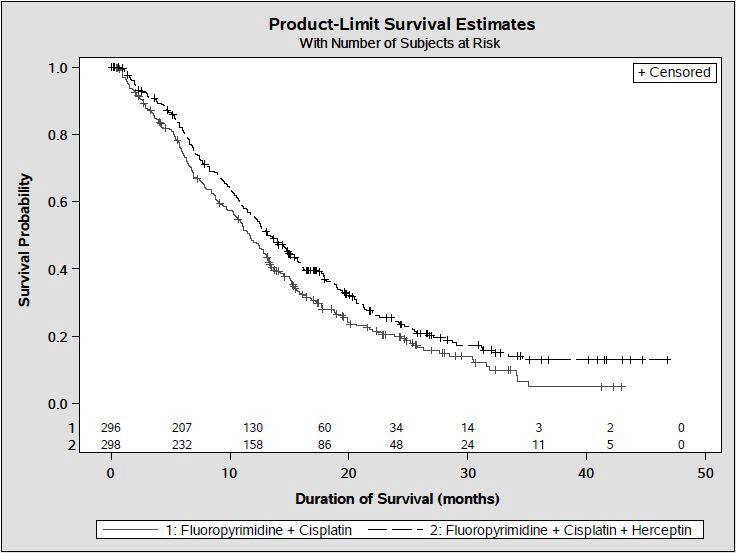
1 INDICATIONS AND USAGE Herceptin is a HER2/neu receptor antagonist indicated for: The treatment of HER2-overexpressing breast cancer. (1.1,1.2) The treatment of HER2 overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma. (1.3) Select patients for therapy based on an FDA-approved companion diagnostic for Herceptin (1, 2.1). 1.1 Adjuvant Breast Cancer Herceptin is indicated for adjuvant treatment of HER2 overexpressing node positive or node negative (ER/PR negative or with one high risk feature [see Clinical Studies (14.1)]) breast cancer as part of a treatment regimen consisting of doxorubicin, cyclophosphamide, and either paclitaxel or docetaxel as part of a treatment regimen with docetaxel and carboplatin as a single agent following multi-modality anthracycline based therapy. Select patients for therapy based on an FDA-approved companion diagnostic for Herceptin [see Dosage and Administration (2.1)]. 1.2 Metastatic Breast Cancer Herceptin is indicated: In combination with paclitaxel for first-line treatment of HER2-overexpressing metastatic breast cancer As a single agent for treatment of HER2-overexpressing breast cancer in patients who have received one or more chemotherapy regimens for metastatic disease. Select patients for therapy based on an FDA-approved companion diagnostic for Herceptin [see Dosage and Administration (2.1)]. 1.3 Metastatic Gastric Cancer Herceptin is indicated, in combination with cisplatin and capecitabine or 5-fluorouracil, for the treatment of patients with HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma who have not received prior treatment for metastatic disease. Select patients for therapy based on an FDA-approved companion diagnostic for Herceptin [see Dosage and Administration (2.1)].
Copyright for images below belongs to manufacturer mentioned in label, presented for information only
Monoclonal antibodies
| Brand Name (Click for details) | Active Ingredient | Drug ATC Class | Price Found (US $) | OVERALL Drug Rating |
|---|